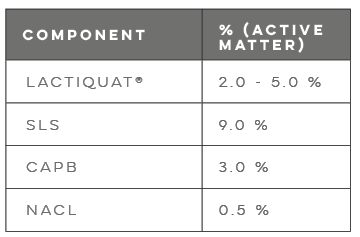
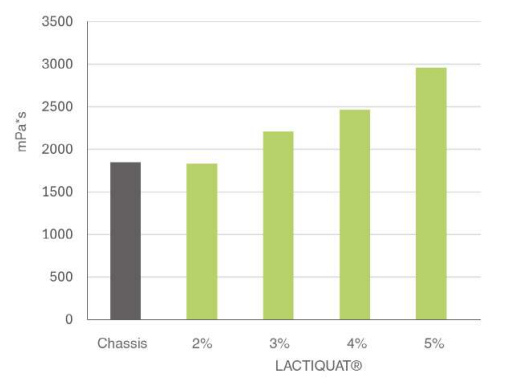
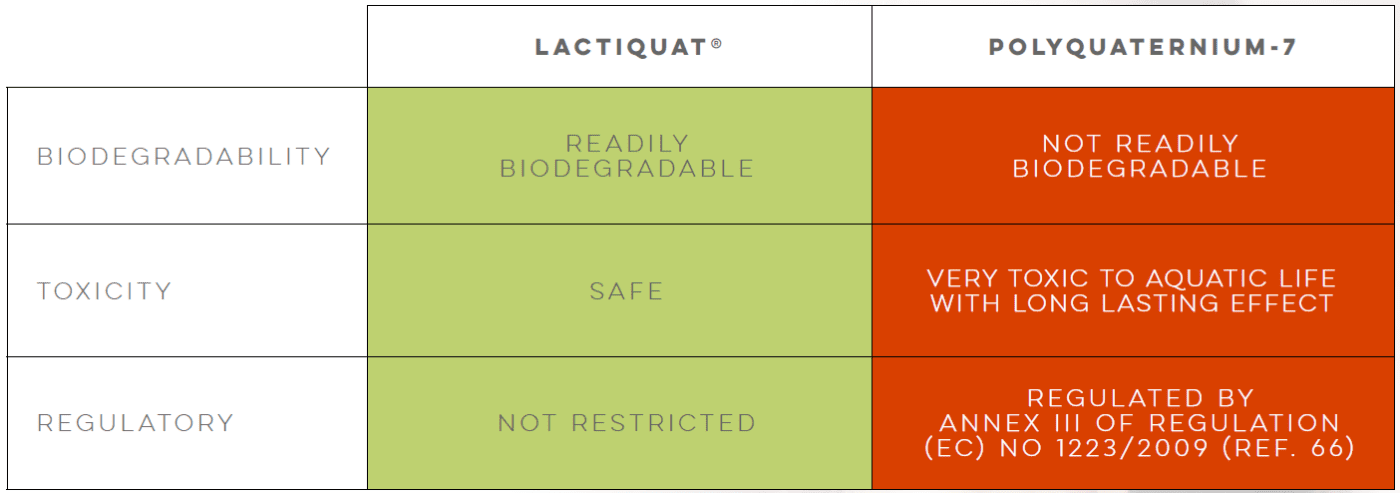
Polyglyceryl-3 Betainate Lactate (LACTIQUAT®) is a 100% plant-derived copolymer with a high cationic charge, derived from betaine and supported by lactic acid, forming a stronger bond with the skin. This formulation can replace synthetic conditioning agents like ammonium chloride and polyquaternium derivatives, improving compatibility and transparency in anionic surfactant systems.
It offers moisturization, smoothness, flexibility, and specific antimicrobial activity, acting as a preservative booster and preventing body odor. Suitable for both rinse-off and leave-on skincare products,
LACTIQUAT® is non-irritating and exhibits maximum cationic charge at pH 4.5-6.5, becoming non-ionic at neutral pH and anionic in alkaline conditions.
KEY COMPONENTS
Betaine, a non-toxic molecule from sugar beet, enhances surfactant compatibility, retains moisture, and improves sensory characteristics. Lactic acid, a milder AHA from sugar beet, exfoliates, improves skin texture, stimulates collagen, promotes a healthy microbiome, and prevents odor.
BETAINE & LACTIC ACID SYNERGISTIC ACTION
SMOOTHING PROPERTIES
LACTIQUAT® blends the hydrating properties of betaine with the gentle exfoliating and skin flexibilty-enhancing effects of lactic acid, resulting in a visible smoothing effect.
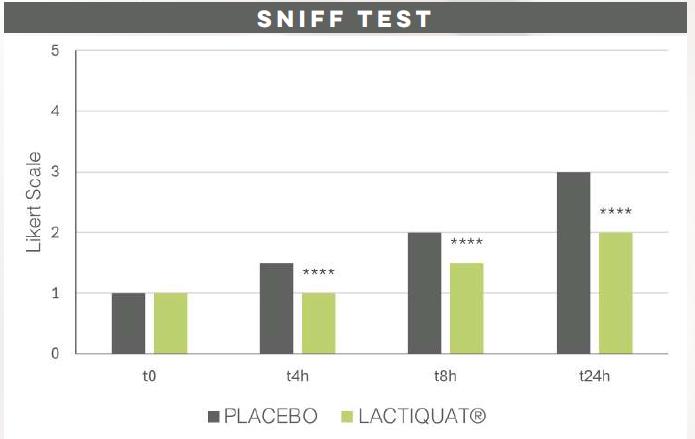
ANTI ODOR EFFICACY
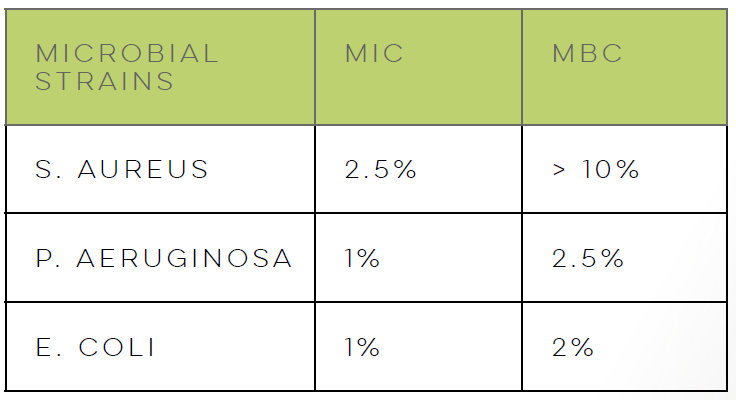
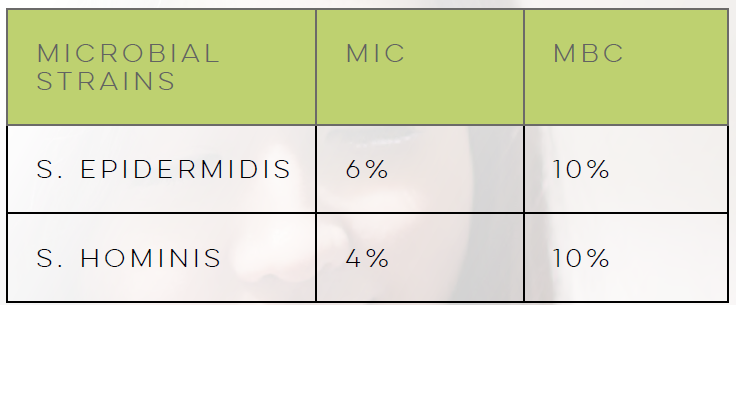
LACTIQUAT® combines the antimicrobial properties of quaternized betaine and lactic acid, effectively serving as a barrier against pathogenic infections, including odor-causing bacteria.
SMOOTHING PROPERTIES
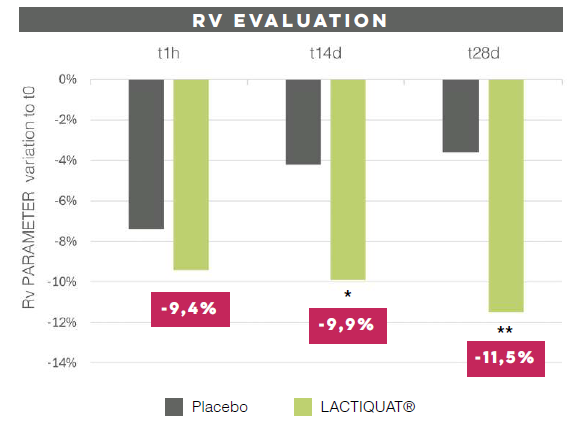
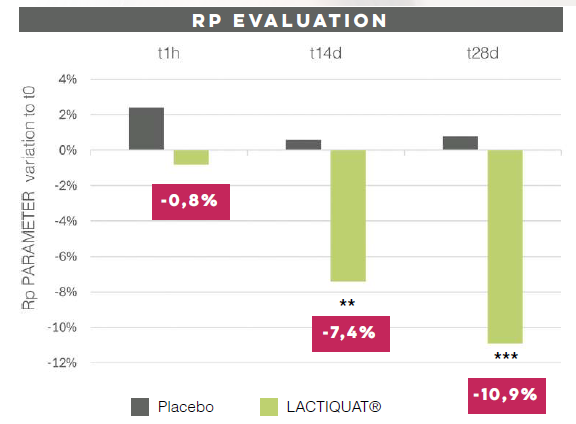
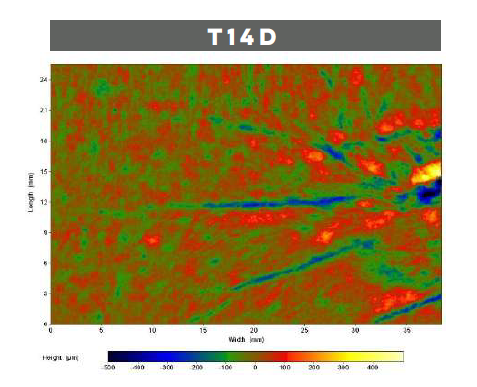
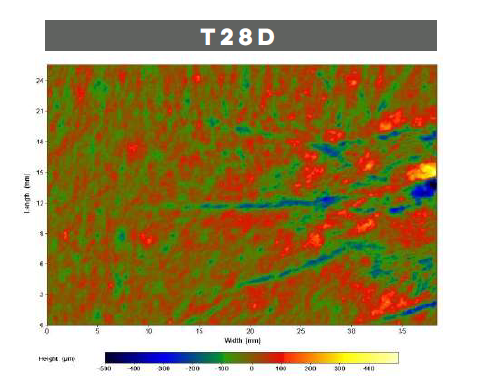
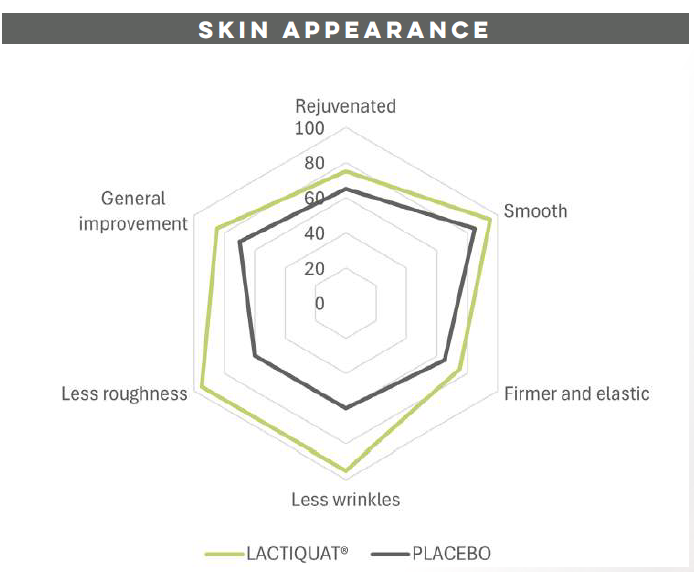
With aging, the cutaneous microrelief, defined as skin irregularities, increases causing a visible roughness and inhomogeneity. LACTIQUAT®, with the synergistic action of betiane and lactic acid, can decrease skin microrelief thus leading to a significant smoothing effect.
To prove that, 4% LACTIQUAT® emulsion* has been tested against a Placebo** on 20 volunteers, aged from 40 to 65, using the “half-face method” (subjects apply each formulation to a different side of the face). Skin moisturization (using CORNEOMETER) and skin profilometry (using PrimosCR SF) have been quantitatively evaluated to assess skin surface properties (hydration, wrinkle depth, volume, roughness, etc).
*LACTIQUAT® face cream: Water, Caprylic/Capric Triglyceride, Lactiquat (Polygyceryl-3 Betainate Lactate), Polyglyceryl-3 Cetyl Ether (and) Sesamum Indicum (Sesame) Seed Oil (and) Malic Acid, Propanediol, Bentonite, Benzyl Alcohol, Caprylyl Glycol, Xanthan Gum.
**Placebo face cream: Water, Caprylic/Capric Triglyceride, Polyglyceryl-3 Cetyl Ether (and) Sesamum Indicum (Sesame) Seed Oil (and) Malic Acid, Propanediol, Bentonite, Benzyl Alcohol, Caprylyl Glycol, Xanthan Gum.
SKIN HYDRATION