Results – Evaluation of the skin irritation potential – GREENQUAT®BT
The obtained results are reported in tables and charts containing the measurements of cell viability, evaluated by MTT assay, on an in vitro 3D reconstructed human epidermis (RHE, SkinEthicTM).
The purpose of the test is to evaluate the possible skin irritation of the products after 24 hours of exposure by assessing the viability of the tissues (RHE, SkinEthic, reconstructed epidermis).
Figure and Table show the graph and corresponding data of cell viability expressed as a percentage of internal positive control (CTRL+, cells treated with SDS) and the product, both compared to the negative control (CTRL-, cells treated with DPBS).
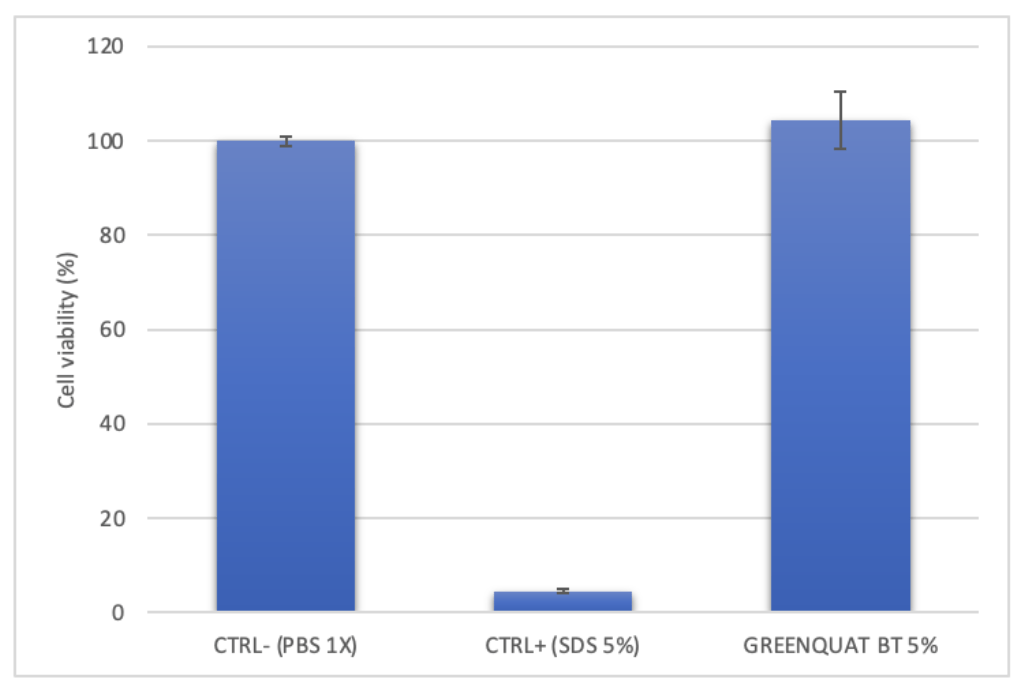
Figure: Effect of 24h exposure with SDS (CTRL+) and the product GREENQUAT®BT 5%, on cell viability (MTT test).
|
Sample |
Cell viability (%) ± standard deviation |
|
CTRL- |
100 ± 0.95 |
|
CTRL+ |
4.50 ± 0.41 |
|
GREENQUAT®BT 5% |
104.40 ± 6.17 |
Table: Percentage changes in cell viability after 24h of treatment with internal positive control (CTRL+) and the tested product respect to negative control (CTRL-, cells treated with DPBS).
The obtained results demonstrate that the product GREENQUAT®BT 5% does not show a cytotoxic effect after 24 hours of contact on 3D in vitro reconstructed human epidermis (RHE, SkinEthicTM), having a cell viability > 50%. Instead, the treatment with SDS (known irritant agent) led to a significant reduction in cell viability compared to CTRL-.
